Let Your Members Know About this Pan-Tumor Trial Information for Advocacy Group Leaders
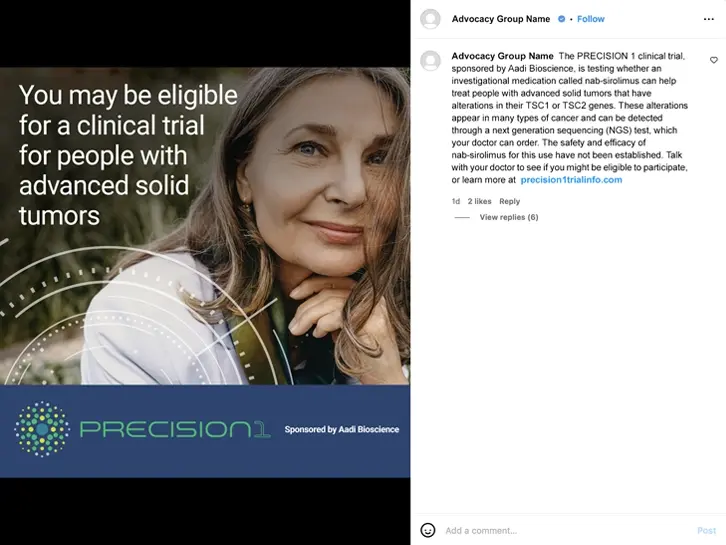
The PRECISION 1 clinical trial is studying the investigational mTOR inhibitor nab-Sirolimus in patients with malignant solid tumors harboring pathogenic inactivating alterations in TSC1 or TSC2 genes.
TSC1 and TSC2 alterations can occur in many solid tumors. A next generation sequencing (NGS) test can identify eligible patients.
The safety and efficacy of nab-sirolimus for this use have not been established.
The materials on this page can help you communicate with your members about this important trial.
Patient Brochure

DOWNLOAD
These materials have been approved by the IRB and can be shared on your social media accounts and other communication channels, such as newsletters, websites, webinars or patient meetings.
Thank you for all you do to support your members through an extremely difficult time in their lives, including by keeping them informed about clinical trials.
If you have any questions about PRECISION 1 or nab-Sirolimus, or if there is anything else we can do to help you help us spread the word about PRECISION 1, please contact us at medinfo@aadibio.com.
Watch the Webinar
Aadi Bioscience recently presented an informational webinar on the PRECISION 1 trial. Watch each video segment of the webinar below.
What is a Basket Trial/Pan-Tumor Study
Why You Should Care About TSC1 and TSC2
How Your Members can Find Out if They Have a TSC1 or TSC2 Mutation
What is PRECISION 1 and How is it Different? (Just-in-Time Model)
How to Participate in PRECISION 1